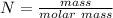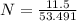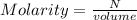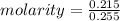Chemistry, 04.04.2020 11:00 lilpump3506
A solution is created by dissolving 11.5 grams of ammonium chloride in enough water to make 255 mL of solution. How many moles of ammonium chloride are present in the resulting solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 23.06.2019 15:30
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2

Chemistry, 23.06.2019 22:00
You are given the reaction cu + hno3> cu(no3)2 + no + h2o
Answers: 1

Chemistry, 24.06.2019 08:50
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? the average kinetic energy remains constant. the average kinetic energy drastically increases. the average kinetic energy drastically decreases. the average kinetic energy gets added to the potential energy.
Answers: 3
You know the right answer?
A solution is created by dissolving 11.5 grams of ammonium chloride in enough water to make 255 mL o...
Questions


Biology, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20


English, 21.01.2021 03:20


Health, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20

Geography, 21.01.2021 03:20

History, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20

History, 21.01.2021 03:20

Arts, 21.01.2021 03:20

Biology, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20



English, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20