
Diluting sulfuric acid with water is highly exothermic: (a) Use Appendix B to find for diluting 1.00 mol of H2SO4(l) (d = 1.83 g/mL) to 1 L of 1.00 M H2SO4(aq) (d = 1.060 g/mL). (b) Suppose you carry out the dilution in a calorimeter. The initial T is 25.0°C, and the specific heat capacity of the final solution is 3.50 J/g·K. What is the final T? (c) Use the ideas of density and heat capacity to explain why you should add acid to water rather than water to acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Diluting sulfuric acid with water is highly exothermic: (a) Use Appendix B to find for diluting 1.00...
Questions

English, 25.04.2021 04:20

History, 25.04.2021 04:20

English, 25.04.2021 04:20

English, 25.04.2021 04:20

Mathematics, 25.04.2021 04:20

Mathematics, 25.04.2021 04:20

Biology, 25.04.2021 04:20



World Languages, 25.04.2021 04:20

Computers and Technology, 25.04.2021 04:20

Mathematics, 25.04.2021 04:20


Social Studies, 25.04.2021 04:20

Mathematics, 25.04.2021 04:20


Chemistry, 25.04.2021 04:20



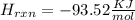
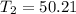 ° c
° c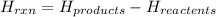
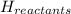 = - 813.9
= - 813.9 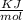
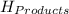 = - 907.51
= - 907.51 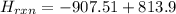
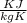
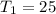 ° c
° c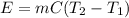
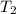 - 298 )
- 298 )

