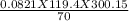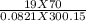A 70-liter tank of oxygen gas at 27 degree Celcius and 42.0 atm springs a leak overnight. when the tank was found in the morning, the pressure in the tank had dropped to 19.0 atm. If the tank originally held 119.4 moles of oxygen gas, how many moles of gas were left the next morning, assuming the temperature and volume of the rank stayed constant?.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
A 70-liter tank of oxygen gas at 27 degree Celcius and 42.0 atm springs a leak overnight. when the t...
Questions

Mathematics, 16.10.2019 15:00



English, 16.10.2019 15:00

History, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00

Geography, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00




Social Studies, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00

Mathematics, 16.10.2019 15:00



Biology, 16.10.2019 15:00