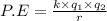Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i. e., largest in magnitude)? Please explain your answer. a.) Na+ - Cl- b.) Na+ - O-2. c.) Al+3 - O-2. d.) Mg+2-O-2 e.) Na- -Mg+2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1
You know the right answer?
Assuming that the distances between the two ions are the same in all cases, which of the following i...
Questions

English, 05.05.2020 21:27





Chemistry, 05.05.2020 21:27

Mathematics, 05.05.2020 21:27


Mathematics, 05.05.2020 21:27



Mathematics, 05.05.2020 21:27



Mathematics, 05.05.2020 21:27

Mathematics, 05.05.2020 21:27




Mathematics, 05.05.2020 21:27