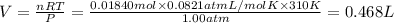Chemistry, 04.04.2020 11:36 kiragor2545
The percent by mass of bicarbonate (HCO3−) in a certain Alka-Seltzer product is 32.5 percent. Calculate the volume of CO2 generated (in mL) at 37°C and 1.00 atm if a person were to accidentally ingest a 3.45-g tablet without following instructions. (Hint: The reaction occurs between HCO3− and HCl acid in the stomach.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
The percent by mass of bicarbonate (HCO3−) in a certain Alka-Seltzer product is 32.5 percent. Calcul...
Questions

Mathematics, 20.05.2021 16:20


Mathematics, 20.05.2021 16:20

History, 20.05.2021 16:20


Mathematics, 20.05.2021 16:20




History, 20.05.2021 16:30





Computers and Technology, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Chemistry, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30

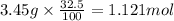
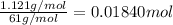
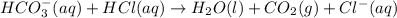
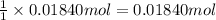 of carbon dioxide gas
of carbon dioxide gas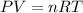 (ideal gas equation)
(ideal gas equation)