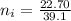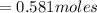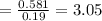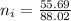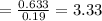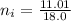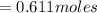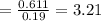Chemistry, 04.04.2020 11:32 rico126090
Now, let's finish the calculation and the determination of the formula of the iron compound: Calculate the % water of hydration : Tries 0/3 Calculate the following for Fe3 : g in 100 g sample mol in 100 g sample mol/mol Fe (3 sig figs) mol/mol Fe (whole number) Tries 0/3 Calculate the following for K : g in 100 g sample mol in 100 g sample mol/mol Fe (3 sig figs) mol/mol Fe (whole number) Tries 0/3 Calculate the following for C2O42-: g in 100 g sample mol in 100 g sample mol/mol Fe (3 sig figs) mol/mol Fe (whole number) Tries 0/3 Calculate the following for H2O g in 100 g sample mol in 100 g sample mol/mol Fe (3 sig figs) mol/mol Fe (whole number)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1



Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Now, let's finish the calculation and the determination of the formula of the iron compound: Calcula...
Questions






Mathematics, 15.11.2019 19:31


English, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31

History, 15.11.2019 19:31

Mathematics, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31

Mathematics, 15.11.2019 19:31

Chemistry, 15.11.2019 19:31


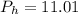 %
%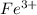 in 100mg is 10.60mg
in 100mg is 10.60mg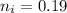
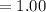
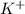 in 100mg is 27.70mg
in 100mg is 27.70mg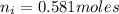
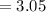
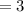
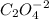 in 100mg is 55.69 mg
in 100mg is 55.69 mg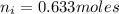
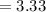
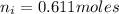
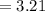
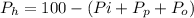
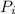 (percentage of iron ) , 22.70% for
(percentage of iron ) , 22.70% for 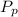 (Percentage of potassium) , 55.69% for
(Percentage of potassium) , 55.69% for 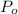 (percentage of Oxlate)
(percentage of Oxlate)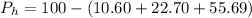
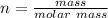
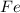 is 55.485 g/mol
is 55.485 g/mol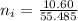
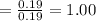
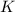 is 39.1 g/mol
is 39.1 g/mol