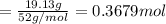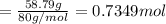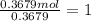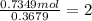Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
A 19.13 gram sample of chromium is heated in the presence of excess bromine. A metal bromide is form...
Questions

Social Studies, 04.03.2021 19:40



Mathematics, 04.03.2021 19:40

English, 04.03.2021 19:40


Mathematics, 04.03.2021 19:40

Chemistry, 04.03.2021 19:40

Mathematics, 04.03.2021 19:40



Mathematics, 04.03.2021 19:40

Social Studies, 04.03.2021 19:40

English, 04.03.2021 19:40


Mathematics, 04.03.2021 19:40


Mathematics, 04.03.2021 19:40

Social Studies, 04.03.2021 19:40

History, 04.03.2021 19:40

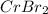 .
.