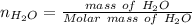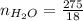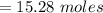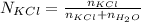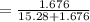Chemistry, 04.04.2020 11:58 avalonr2003
A solution was prepared by dissolving 125.0 g of KCl in 275 g of water. Calculate the mole fraction of KCl. (The formula weight of KCl is 74.6 g/mol. The formula weight of water is 18.0 g/mol.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
A solution was prepared by dissolving 125.0 g of KCl in 275 g of water. Calculate the mole fraction...
Questions


Mathematics, 06.02.2021 01:40

History, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40


Geography, 06.02.2021 01:40




Mathematics, 06.02.2021 01:40


Mathematics, 06.02.2021 01:40



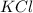 is
is 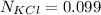
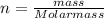
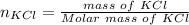
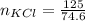
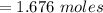
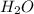 is
is