
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: (g)(g)(g) In the second step, ammonia and oxygen react to form nitric acid and water: (g)(g)(g)(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions

Advanced Placement (AP), 27.12.2019 16:31

Mathematics, 27.12.2019 16:31


Mathematics, 27.12.2019 16:31


Mathematics, 27.12.2019 16:31


English, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

English, 27.12.2019 16:31


Physics, 27.12.2019 16:31



Mathematics, 27.12.2019 16:31

History, 27.12.2019 16:31

History, 27.12.2019 16:31


Mathematics, 27.12.2019 16:31

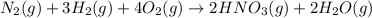
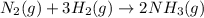 (1)
(1)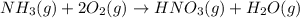
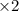
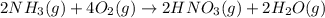 (2)
(2)

