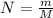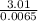Chemistry, 04.04.2020 12:39 Savageman4654
In an experiment to determine the enthalpy change for this reaction, you combine 0.158 g of Mg metal with enough HCl to make 100.0 mL of solution in a coffee-cup calorimeter. The HCl is sufficiently concentrated so that the Mg completely reacts. The temperature of the solution rises from 25.6 °C to 32.8 °C as a result of the reaction. Find ΔHrxn for the reaction as written. Use 1.00 g/mL as the dens

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1


Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
In an experiment to determine the enthalpy change for this reaction, you combine 0.158 g of Mg metal...
Questions


Computers and Technology, 06.11.2020 21:50

Mathematics, 06.11.2020 21:50


Chemistry, 06.11.2020 21:50


Mathematics, 06.11.2020 21:50

Mathematics, 06.11.2020 21:50


Mathematics, 06.11.2020 21:50

Mathematics, 06.11.2020 21:50

Biology, 06.11.2020 21:50

Mathematics, 06.11.2020 21:50


Mathematics, 06.11.2020 21:50

Health, 06.11.2020 21:50

English, 06.11.2020 21:50



Mathematics, 06.11.2020 22:00

 for the reaction = 463
for the reaction = 463 

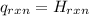
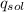 ------ (1)
------ (1)
 (1)(100) × 4.18 × (32.8 - 25.6)
(1)(100) × 4.18 × (32.8 - 25.6)