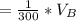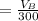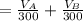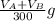Chemistry, 04.04.2020 14:09 vladsmolin7781
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your crystallization and washing with water were done at this temperature, what weight of aspirin did you lose in the filtrate and washings? How much was your percent yield lowered by this loss?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

You know the right answer?
The solubility of aspirin in water is 1 g per 300 mL at 25 degrees celsius. Assuming that your cryst...
Questions

Spanish, 31.08.2019 07:50


Mathematics, 31.08.2019 07:50


Biology, 31.08.2019 07:50


Mathematics, 31.08.2019 07:50


Mathematics, 31.08.2019 07:50

Chemistry, 31.08.2019 07:50

English, 31.08.2019 07:50


Mathematics, 31.08.2019 07:50


Biology, 31.08.2019 07:50


Mathematics, 31.08.2019 07:50

English, 31.08.2019 07:50

Mathematics, 31.08.2019 07:50

Biology, 31.08.2019 07:50

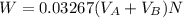
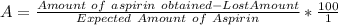
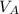
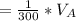
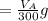
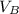 to wash the crystallized aspirin then the lost during washing would be
to wash the crystallized aspirin then the lost during washing would be