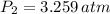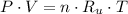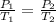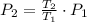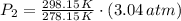Chemistry, 04.04.2020 20:16 dogsarecute278
Air was put into an automobile tire when the tire was cold, at 5.00 C. The tire's air pressure was 3.04 atm. Later, the weather warms up. Also, the automobile is driven, further warming the tire. What will the tire's pressure be when the temperature is 25.0 C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Air was put into an automobile tire when the tire was cold, at 5.00 C. The tire's air pressure was 3...
Questions


Mathematics, 02.07.2019 19:30


Mathematics, 02.07.2019 19:30


Computers and Technology, 02.07.2019 19:30




Biology, 02.07.2019 19:30


Physics, 02.07.2019 19:30


Mathematics, 02.07.2019 19:30

Physics, 02.07.2019 19:30


Mathematics, 02.07.2019 19:30



Mathematics, 02.07.2019 19:30