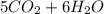Chemistry, 04.04.2020 19:45 gibbss80stu
At 500.K and 1.00 atm pressure, 1.5 liters of pentane gas, C5H12, is mixed with 15 liters of oxygen gas. A complete combustion results. How many liters of WATER vapor, measured at the same temperature and pressure, would be produced after the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
At 500.K and 1.00 atm pressure, 1.5 liters of pentane gas, C5H12, is mixed with 15 liters of oxygen...
Questions







History, 23.07.2019 17:00



History, 23.07.2019 17:00

Chemistry, 23.07.2019 17:00

History, 23.07.2019 17:00





Advanced Placement (AP), 23.07.2019 17:00



Mathematics, 23.07.2019 17:00

 →
→