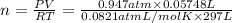Chemistry, 04.04.2020 21:02 kevinhill185
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is produced by water displacement. If the lab temperature is 24 C and the atmospheric pressure is 742.1 mm Hg, how many grams of hydrogen are produced? Water vapor pressure is 22.4 mm Hg at 24 C.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If 0.414 g of hydrogen is obtained in this experiment how many grams of sulfur must be obtained
Answers: 2

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

You know the right answer?
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is...
Questions



Mathematics, 12.03.2021 01:10



Mathematics, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10


Business, 12.03.2021 01:10

Mathematics, 12.03.2021 01:10



History, 12.03.2021 01:10

Arts, 12.03.2021 01:10


Mathematics, 12.03.2021 01:20

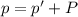
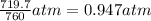
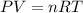 ( Ideal gas equation)
( Ideal gas equation)