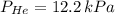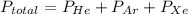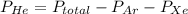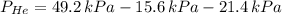Chemistry, 04.04.2020 21:30 maisonsuperman5321
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure of helium if the total pressure is 49.2 kPa and the partial pressure of Ar is 15.6 kPa and 21,4 kPa for Xe?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure...
Questions

History, 10.02.2020 22:28



English, 10.02.2020 22:28


Social Studies, 10.02.2020 22:28





Biology, 10.02.2020 22:28


Mathematics, 10.02.2020 22:28

Health, 10.02.2020 22:28

Mathematics, 10.02.2020 22:28

English, 10.02.2020 22:28

Computers and Technology, 10.02.2020 22:28