
Chemistry, 04.04.2020 21:52 Shybaby5019
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 521 g of NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

You know the right answer?
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution conta...
Questions





Computers and Technology, 06.07.2019 18:30

Mathematics, 06.07.2019 18:30

Chemistry, 06.07.2019 18:30

Computers and Technology, 06.07.2019 18:30

Chemistry, 06.07.2019 18:30

Chemistry, 06.07.2019 18:30







Mathematics, 06.07.2019 18:30

Biology, 06.07.2019 18:30


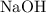 solution is approximately
solution is approximately 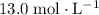 .
.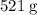 of
of 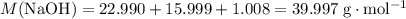 .
.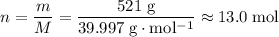 .
.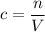 to find the molar concentration
to find the molar concentration  of this solution. In this equation,
of this solution. In this equation, 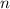 is the number of moles of the solute, and
is the number of moles of the solute, and 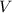 is the volume of the solution.
is the volume of the solution.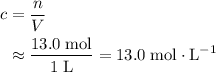 .
. 

