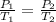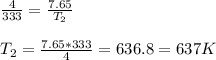Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
The gas begins with a temperature of 333 K and a pressure of 4.00 atm that increases to 7.65 atm as...
Questions

Mathematics, 14.07.2019 05:30

Mathematics, 14.07.2019 05:30


English, 14.07.2019 05:30



History, 14.07.2019 05:30

English, 14.07.2019 05:30


Arts, 14.07.2019 05:30

Chemistry, 14.07.2019 05:30


Mathematics, 14.07.2019 05:30

Mathematics, 14.07.2019 05:30


Mathematics, 14.07.2019 05:30