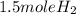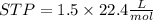Chemistry, 05.04.2020 05:17 screamqueen
2Li + H2SO4=Li2SO4 + H2 How many liters of hydrogen gas, H2 at STP can be produced from 3.0 moles of Li? The molar volume of a gas at STP is 22.4L/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
2Li + H2SO4=Li2SO4 + H2 How many liters of hydrogen gas, H2 at STP can be produced from 3.0 moles of...
Questions

English, 26.02.2021 22:00

English, 26.02.2021 22:00


Mathematics, 26.02.2021 22:00

Mathematics, 26.02.2021 22:00




Spanish, 26.02.2021 22:00

Biology, 26.02.2021 22:00



Mathematics, 26.02.2021 22:00

Mathematics, 26.02.2021 22:00


Mathematics, 26.02.2021 22:00

Mathematics, 26.02.2021 22:00



Mathematics, 26.02.2021 22:00

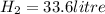
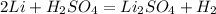
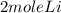 ⇔
⇔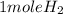
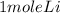 ⇔
⇔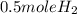
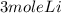 ⇔
⇔