

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
If 5.3 g of gallium reactions with 5.3 g of oxygen according to the following reaction, how
man...
man...
Questions

Mathematics, 03.06.2021 01:40

Mathematics, 03.06.2021 01:40


Mathematics, 03.06.2021 01:40

Mathematics, 03.06.2021 01:40


Mathematics, 03.06.2021 01:40




Mathematics, 03.06.2021 01:40





Mathematics, 03.06.2021 01:40


English, 03.06.2021 01:40


Mathematics, 03.06.2021 01:40

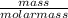
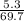 = 0.08mole
= 0.08mole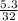 = 0.17mole
= 0.17mole 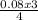 = 0.06 moles of O₂
= 0.06 moles of O₂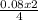 = 0.04moles
= 0.04moles 

