

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


You know the right answer?
The enthalpy change for converting 10.0 g of ice at -25.0 °c to water at 90.0 °c is kj. the specifi...
Questions


Mathematics, 06.11.2020 14:00

English, 06.11.2020 14:00

Biology, 06.11.2020 14:00

English, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00





Social Studies, 06.11.2020 14:00



Mathematics, 06.11.2020 14:00

Computers and Technology, 06.11.2020 14:00


Business, 06.11.2020 14:00

Arts, 06.11.2020 14:00

Biology, 06.11.2020 14:00

English, 06.11.2020 14:00

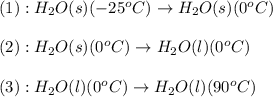
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0474/9125/5cd06.png)
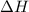 = enthalpy change = ?
= enthalpy change = ?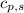 = specific heat of solid water =
= specific heat of solid water = 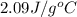
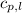 = specific heat of liquid water =
= specific heat of liquid water = 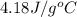
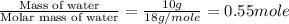
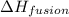 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 4.18J/gK\times (0-(-25))^oC]+0.55mole\times 6010J/mole+[10g\times 2.09J/gK\times (90-0)^oC]](/tpl/images/0474/9125/4de79.png)
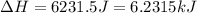 (1 KJ = 1000 J)
(1 KJ = 1000 J)

