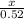Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
If 3.18 x 10^23 atoms of iron react with 67.2 L of chlorine gas at STP, what is the maximum
num...
num...
Questions



Mathematics, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50



Mathematics, 09.10.2019 08:50


Mathematics, 09.10.2019 08:50

Geography, 09.10.2019 08:50


History, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50

English, 09.10.2019 08:50


English, 09.10.2019 08:50


Mathematics, 09.10.2019 08:50

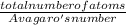
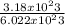
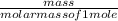
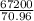 (atomic mass of chlorine gas = 70.96 grams/mole)
(atomic mass of chlorine gas = 70.96 grams/mole)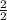 =
=