
Chemistry, 06.04.2020 11:30 justice808
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at the same conditions of temperature and pressure.
2N2(g) + 3O2(g) 2N2O3(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide...
Questions

Mathematics, 27.05.2020 23:00

Mathematics, 27.05.2020 23:00



Spanish, 27.05.2020 23:00


Mathematics, 27.05.2020 23:00


Mathematics, 27.05.2020 23:00

Mathematics, 27.05.2020 23:00

Mathematics, 27.05.2020 23:00

History, 27.05.2020 23:00

Mathematics, 27.05.2020 23:00

Mathematics, 27.05.2020 23:00



Mathematics, 27.05.2020 23:00


Mathematics, 27.05.2020 23:00

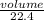
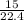 = 0.67moles
= 0.67moles 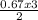 = 1.01 moles of O₂
= 1.01 moles of O₂

