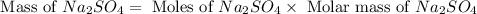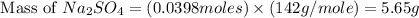Chemistry, 06.04.2020 16:59 zitterkoph
Aqueous sulfuric acid (H2SO4) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na2SO4) and liquid water (H2O). What is the theoretical yield of sodium sulfate formed from the reaction of 3.9 g of sulfuric acid and 1.8g of sodium hydroxide?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Aqueous sulfuric acid (H2SO4) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium su...
Questions


Biology, 07.12.2020 09:40


Mathematics, 07.12.2020 09:40

Mathematics, 07.12.2020 09:40


English, 07.12.2020 09:40

Mathematics, 07.12.2020 09:40

Social Studies, 07.12.2020 09:40

English, 07.12.2020 09:40

Biology, 07.12.2020 09:40

Mathematics, 07.12.2020 09:40

Medicine, 07.12.2020 09:40




English, 07.12.2020 09:50

English, 07.12.2020 09:50


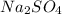 is, 5.65 grams.
is, 5.65 grams.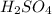 = 3.9 g
= 3.9 g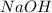 = 7.8 g
= 7.8 g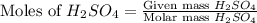
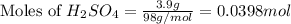
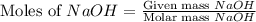
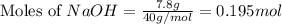
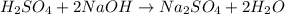
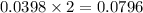 moles of
moles of