
Chemistry, 06.04.2020 18:02 wilkoASK2919
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially contains 4.0 atm of gas (PA = 2.0 atm and PB = 2.0 atm).
Which statement is true of the reaction mixture?
(a) The reaction mixture will proceed toward products.
(b) The reaction mixture is at equilibrium.
(c) The reaction mixture will proceed toward reactants
(d) It is not possible to determine from the information given the future direction of the reaction mixture

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
During metamorphic processes, increased pressure and temperature can affect the of minerals in rock. rocks subjected to very high pressure are typically than others because mineral grains are squeezed together, and the atoms are more closely packed. during metamorphic processes, water facilitates the transfer of ions between and within minerals, which can the rate at which metamorphic reactions take place. the growth of new minerals within a rock during metamorphism has been estimated to be about per million years. metamorphism is commonly associated with convergent plate boundaries, where two plates move toward each other. during contact metamorphism, a large intrusion will contain thermal energy and will cool much more slowly than a small one. metamorphosed sandstone is known as the metamorphic rock made from metamorphosed shale, was once used to make blackboards for classrooms.
Answers: 1

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
You know the right answer?
For the reaction 2A(g) â B(g), the equilibrium constant is Kp = 0.76. A reaction mixture initially c...
Questions

Mathematics, 26.03.2021 08:10


Mathematics, 26.03.2021 08:10

Chemistry, 26.03.2021 08:10



Mathematics, 26.03.2021 08:10

Social Studies, 26.03.2021 08:10


English, 26.03.2021 08:10



History, 26.03.2021 08:10

Mathematics, 26.03.2021 08:10

English, 26.03.2021 08:20

Chemistry, 26.03.2021 08:20


History, 26.03.2021 08:20


History, 26.03.2021 08:20

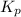
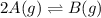
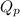 is written as:
is written as: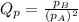
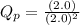
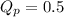
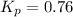
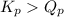 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.

