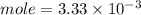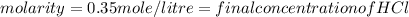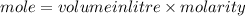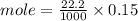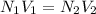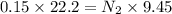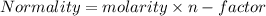Chemistry, 06.04.2020 18:02 hntnhtthnyt
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of unknown concentration. HCl is dispensed into the reaction flask and Phenolphthalein is added as an indicator. At the end of the titration, 22.2ml of NaOH and 9.45ml HCl were used.
Required:
A. Write a balanced equation for the Acid-Base reaction.
B. What is the total volume of NaOH used in the titration?
C. How many moles of NaOH was used?
D. What is the total volume of HCl used in the titration?
E. What is the final concentration of the HCl solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of un...
Questions

English, 01.02.2021 17:50

Arts, 01.02.2021 17:50





Medicine, 01.02.2021 17:50



Mathematics, 01.02.2021 17:50

History, 01.02.2021 17:50

History, 01.02.2021 17:50


Advanced Placement (AP), 01.02.2021 17:50


Mathematics, 01.02.2021 17:50


History, 01.02.2021 17:50

Mathematics, 01.02.2021 17:50