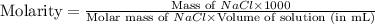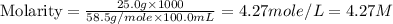Chemistry, 06.04.2020 18:19 Gghbhgy8716
25.0g of NaCl is placed in a 100.0ml volumetric flask. Enough water is added to dissolve the salt and then the volume is brought to 100.0ml total. What is the molar (moles/L or M) concentration of the NaCl?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
25.0g of NaCl is placed in a 100.0ml volumetric flask. Enough water is added to dissolve the salt an...
Questions

Biology, 07.07.2019 10:30

Mathematics, 07.07.2019 10:30

Biology, 07.07.2019 10:30



Mathematics, 07.07.2019 10:30

Biology, 07.07.2019 10:30





Mathematics, 07.07.2019 10:30

Biology, 07.07.2019 10:30

Health, 07.07.2019 10:30


Mathematics, 07.07.2019 10:30

Social Studies, 07.07.2019 10:30


History, 07.07.2019 10:30

Mathematics, 07.07.2019 10:30

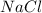 in the solution is, 4.27 M
in the solution is, 4.27 M