Chemistry, 06.04.2020 18:21 roseemariehunter12
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g).
(a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g).
(b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
(c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) becaus...
Questions


Mathematics, 12.01.2021 06:40


History, 12.01.2021 06:40


Mathematics, 12.01.2021 06:40

Geography, 12.01.2021 06:40

Mathematics, 12.01.2021 06:40


Mathematics, 12.01.2021 06:40

Mathematics, 12.01.2021 06:40

Engineering, 12.01.2021 06:40


Physics, 12.01.2021 06:40

Chemistry, 12.01.2021 06:40

Computers and Technology, 12.01.2021 06:40




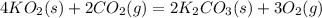
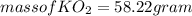 and
and 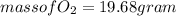
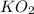 need 2 moles of
need 2 moles of 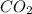 for complete reaction i.e. mole
for complete reaction i.e. mole 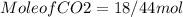
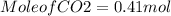
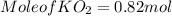
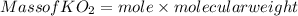
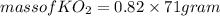
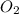 is produced
is produced 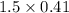 mole of
mole of