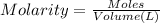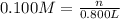Chemistry, 06.04.2020 18:54 devinmoore4664
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
She will do this in three steps:
Fill a 800 ml volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

You know the right answer?
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
Questions

Biology, 18.09.2019 05:30


Mathematics, 18.09.2019 05:30











English, 18.09.2019 05:30

Business, 18.09.2019 05:30


World Languages, 18.09.2019 05:30



Computers and Technology, 18.09.2019 05:30

![pOH=-\log[OH^-]](/tpl/images/0584/6258/fe336.png)
![1.00=-\log[OH^-]](/tpl/images/0584/6258/6b8f7.png)
![[OH^-]=10^{-1.00} M=0.100 M](/tpl/images/0584/6258/a9d8c.png)
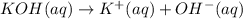
![[KOH]=[OH^-]=[K^+]=0.100 M](/tpl/images/0584/6258/fca0f.png)