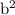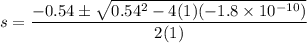Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8 × 10−10.
Calculate the value of [Ag+] in a saturated solution of AgCl in distilled water.
The concentration of Cl−(aq) in seawater is 0.54 M.
Calculate the molar solubility of AgCl(s) in seawater.
Explain why AgCl(s) is less soluble in seawater than in distilled water.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8...
Questions


Mathematics, 05.11.2020 23:00

Biology, 05.11.2020 23:00



Mathematics, 05.11.2020 23:00

Biology, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00


Health, 05.11.2020 23:00


Social Studies, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00

Physics, 05.11.2020 23:00




Engineering, 05.11.2020 23:00


 M.
M.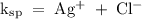
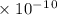 = x
= x  x
x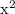

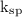 = b
= b