
Chemistry, 06.04.2020 22:19 birdwithpurpleboots
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.09 L. If the combustion of this mixture releases 900. Jof energy, to what volume will the gases expand against a constant pressure of 670. torr if all the energy of combustion is converted into work to push back the piston? (1 atm = 760 torr, 1 L atm = 101.325 J) a. 10.00 L b. 10.17 L c. 1.47 L d. 1.34L

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.0...
Questions



Mathematics, 09.12.2020 21:20

Arts, 09.12.2020 21:20

Spanish, 09.12.2020 21:20



Biology, 09.12.2020 21:20


English, 09.12.2020 21:20

Mathematics, 09.12.2020 21:20


Mathematics, 09.12.2020 21:20



Chemistry, 09.12.2020 21:20


Mathematics, 09.12.2020 21:20


Mathematics, 09.12.2020 21:20

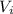 = 0.09 L, P = 670 torr
= 0.09 L, P = 670 torr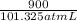
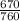 atm
atm
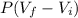
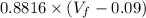
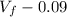 = 10.08
= 10.08
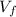 = 10.17 L
= 10.17 L

