Chemistry, 06.04.2020 23:45 markarianlaura1
2- A sheet of steel 3.0-mm thick has nitrogen atmospheres on both sides at 900°C and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 1.85 à 10â10 m2/s, and the diffusion flux is found to be 1.0 à 10â7 kg/m2 . s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 2 kg/m3. How far into the sheet from this high-pressure side will the concentration be 0.5 kg/m3? Assume a linear concentration profile. (40 pts.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2



Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
2- A sheet of steel 3.0-mm thick has nitrogen atmospheres on both sides at 900°C and is permitted t...
Questions




Mathematics, 19.12.2019 18:31




Social Studies, 19.12.2019 18:31


Mathematics, 19.12.2019 18:31






Biology, 19.12.2019 18:31




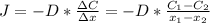
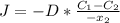 (1)
(1)