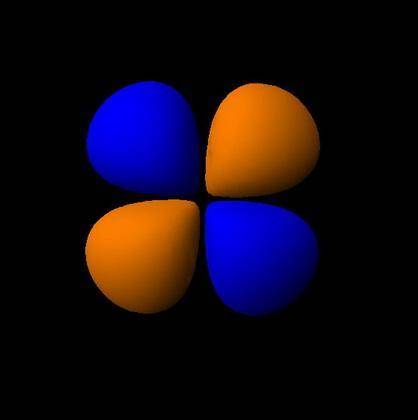Chemistry, 07.04.2020 00:47 glowbaby123
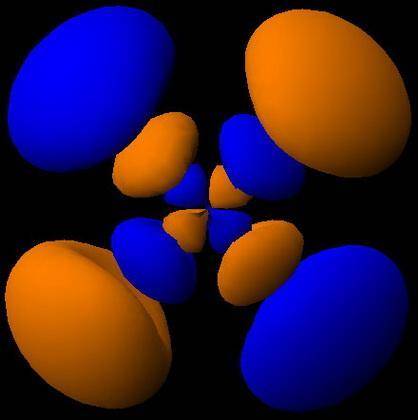
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Determine which statement is true or false.
The orbital in the n = 5 shell is bigger than the orbital in the n = 3 shell.
The value of l would increase by 2 for the n = 5 shell.
The value of l for both orbitals would be the same.
The orientation of the n = 5 orbital would be rotated 45∘ along the xy plane.
The mâ„“ value for both orbitals would be the same.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

You know the right answer?
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Questions

















Health, 21.03.2020 02:05



Social Studies, 21.03.2020 02:05