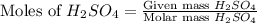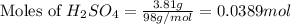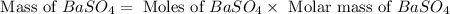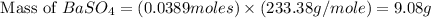Chemistry, 07.04.2020 01:26 eaalvarezelisa01
For the following reaction, 3.81 grams of sulfuric acid are mixed with excess barium hydroxide. The reaction yields 8.45 grams of barium sulfate.
barium hydroxide (aq) + sulfuric acid (aq) ---> barium sulfate (s) + water (l)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
For the following reaction, 3.81 grams of sulfuric acid are mixed with excess barium hydroxide. The...
Questions

Health, 17.01.2020 15:31



Mathematics, 17.01.2020 15:31


English, 17.01.2020 15:31

History, 17.01.2020 15:31

History, 17.01.2020 15:31





Geography, 17.01.2020 15:31





English, 17.01.2020 15:31

English, 17.01.2020 15:31

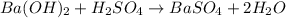
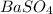 is, 9.08 grams.
is, 9.08 grams.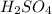 = 3.81 g
= 3.81 g