Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of t...

Chemistry, 07.04.2020 02:36 maddysmall32
Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of this reaction at a certain temperature was found to have
[CO]=0.115M, [H2]=0.116M, and [CH3OH]=0.190M. What is the value of the equilibrium constant (Kc) at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Questions


Spanish, 12.02.2020 19:21

Mathematics, 12.02.2020 19:21

Mathematics, 12.02.2020 19:21


Mathematics, 12.02.2020 19:21


Social Studies, 12.02.2020 19:21




Mathematics, 12.02.2020 19:22

History, 12.02.2020 19:22







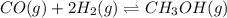
![[CO]=0.115 M,[H_2]=0.116 M](/tpl/images/0585/7114/6fdc2.png)
![[CH_3OH]=0.190 M](/tpl/images/0585/7114/98ee3.png)
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0585/7114/4cf94.png)




