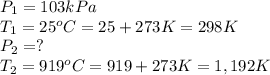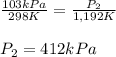1. The gas in a used aerosol can is at a pressure 103 kPa at 25°C. If the can
is thrown into a...

Chemistry, 07.04.2020 03:14 SallyMarquez1201
1. The gas in a used aerosol can is at a pressure 103 kPa at 25°C. If the can
is thrown into a fire, what will the pressure be when the temperature reaches
919°C?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Questions



Physics, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10


Chemistry, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10


Mathematics, 20.12.2020 04:10

English, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10

Computers and Technology, 20.12.2020 04:10

Biology, 20.12.2020 04:10


Mathematics, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10

Mathematics, 20.12.2020 04:10


Mathematics, 20.12.2020 04:10

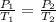
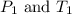 are the initial pressure and temperature of the gas in a used aerosol can.
are the initial pressure and temperature of the gas in a used aerosol can.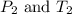 are the final pressure and temperature of the gas in a used aerosol can.
are the final pressure and temperature of the gas in a used aerosol can.