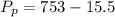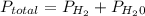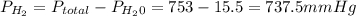Chemistry, 07.04.2020 03:19 blueyish6422
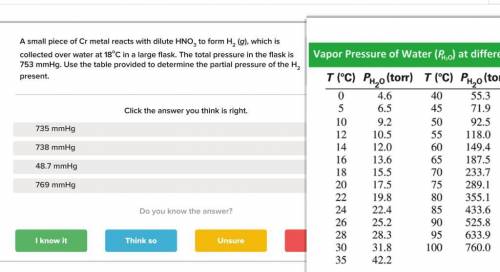
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 18 C in a large flask. The total pressure in the flask is 753 mmHg.
Determine the partial pressure of the H2 present.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 1...
Questions


Mathematics, 11.01.2021 07:40


Business, 11.01.2021 07:40

Mathematics, 11.01.2021 07:40


Mathematics, 11.01.2021 07:40


Mathematics, 11.01.2021 07:40

Mathematics, 11.01.2021 07:40

History, 11.01.2021 07:40

Mathematics, 11.01.2021 07:40



Mathematics, 11.01.2021 07:40

English, 11.01.2021 07:40



Spanish, 11.01.2021 07:40

History, 11.01.2021 07:40

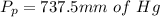
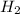 is mathematically represented as
is mathematically represented as 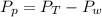
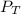 is the total pressure of water with a value of 15.5 mm of Hg
is the total pressure of water with a value of 15.5 mm of Hg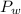 is the partial pressure of water with a value 753 mm of Hg
is the partial pressure of water with a value 753 mm of Hg