
Chemistry, 07.04.2020 04:13 crarylolmeow
HBrO(aq) + H2O(l) ⇄ H3O+(aq) + BrO−(aq) Keq=2.8×10−9
The equilibrium reaction in 0.100M HBrO(aq) at equilibrium is represented by the equation above. Based on the magnitude of the equilibrium constant, which of the following correctly compares the equilibrium concentrations of substances involved in the reaction, and why?
answer choices
The equilibrium concentration of BrO− will be much smaller than the equilibrium concentration of H3O+, because H2O is the solvent and is present in the largest amount.
The equilibrium concentration of BrO− will be much smaller than the equilibrium concentration of HBrO, because Keq<<1
The equilibrium concentration of H3O+ will be much smaller than the equilibrium concentration of BrO−, because all the HBrO will react to produce BrO−
The equilibrium concentration of H3O+ will be much larger than the equilibrium concentration of HBrO, because Keq<<1.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
HBrO(aq) + H2O(l) ⇄ H3O+(aq) + BrO−(aq) Keq=2.8×10−9
The equilibrium reaction in 0.100M...
The equilibrium reaction in 0.100M...
Questions

Mathematics, 25.03.2021 21:00

Mathematics, 25.03.2021 21:00




World Languages, 25.03.2021 21:00

Mathematics, 25.03.2021 21:00



Social Studies, 25.03.2021 21:00

Mathematics, 25.03.2021 21:00


Chemistry, 25.03.2021 21:00





Mathematics, 25.03.2021 21:00

Mathematics, 25.03.2021 21:00

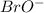 will be much smaller than the equilibrium concentration of
will be much smaller than the equilibrium concentration of 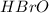 , because Keq<<1
, because Keq<<1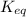
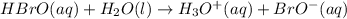
![K=\frac{[H_3O^+]\times [BrO^-]}{[HBrO]}](/tpl/images/0585/9548/ee59c.png)
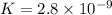
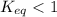 , That means the concentration of products is less as the reaction does not proceed much towards the forward direction.
, That means the concentration of products is less as the reaction does not proceed much towards the forward direction.

