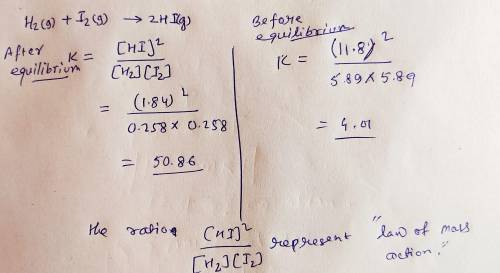
Onsider the chemical system: H2(g)+I2(g)>2HI(g)
5.89 mol H2, 5.89 mol I2, and 11.8 mo...

Chemistry, 07.04.2020 04:59 decoreyjpaipxv
Onsider the chemical system: H2(g)+I2(g)>2HI(g)
5.89 mol H2, 5.89 mol I2, and 11.8 mol HI are placed in a closed 10.00 L container maintained at 448°C. At equilibrium, the concentrations of H2, I2, and HI are 0.258 , 0.258 , and 1.84 M.
A. The ratio
[HI]2 / [H2][I2]
represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.
B. The value, 4.01 , represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.
C. The value, 50.9 , represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Questions


Mathematics, 14.12.2020 09:40




Mathematics, 14.12.2020 09:40

Chemistry, 14.12.2020 09:40

Mathematics, 14.12.2020 09:40

Chemistry, 14.12.2020 09:40

Advanced Placement (AP), 14.12.2020 09:40


Mathematics, 14.12.2020 09:40


Geography, 14.12.2020 09:40




Mathematics, 14.12.2020 09:40

Mathematics, 14.12.2020 09:40

![\frac{[HI]^{2} }{[H_{2}[I_{2} ] }](/tpl/images/0586/0737/3585a.png) represent
represent