Chemistry, 07.04.2020 04:53 granthazenp5e9mj
A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
What is the molar mass of the acid if 19.0 mL of the KOH solution is required to neutralize the sample?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1


Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
Wh...
Wh...
Questions





Biology, 30.03.2021 20:40

Arts, 30.03.2021 20:40

Arts, 30.03.2021 20:40







Mathematics, 30.03.2021 20:40


Mathematics, 30.03.2021 20:40

Mathematics, 30.03.2021 20:40

Mathematics, 30.03.2021 20:40

Mathematics, 30.03.2021 20:40

Mathematics, 30.03.2021 20:40

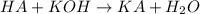
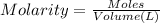
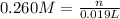
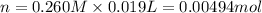
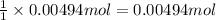 of HA
of HA