
Chemistry, 07.04.2020 05:32 kaitlksndj
Given the reaction:
HCl(g) + H2O(l) → H3O+(aq) + Cl−(aq)
Which reactant acted as a Bronsted-Lowry acid?
A) H2O(l), because it accepted protons
B) H2O(l), because it produced hydronium ions C) HCl(g), because it donated protons
D) HCl(g), because it reacted with chloride ions

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
Given the reaction:
HCl(g) + H2O(l) → H3O+(aq) + Cl−(aq)
Which reactant acted as a Brons...
HCl(g) + H2O(l) → H3O+(aq) + Cl−(aq)
Which reactant acted as a Brons...
Questions



Law, 17.03.2021 23:50



Chemistry, 17.03.2021 23:50


English, 17.03.2021 23:50

Geography, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Business, 17.03.2021 23:50

Chemistry, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

History, 17.03.2021 23:50



History, 17.03.2021 23:50

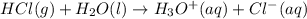
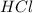 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms 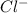 which is a conjugate base.
which is a conjugate base.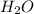 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms 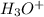 which is a conjugate acid.
which is a conjugate acid.

