*WILL GIVE BRAINLIEST IF SOMEONE ANSWERS THIS CORRECTLY ASAP*
The contents of two beaker...

*WILL GIVE BRAINLIEST IF SOMEONE ANSWERS THIS CORRECTLY ASAP*
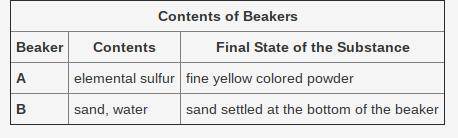
The contents of two beakers are described in the table.
*Table Inserted Below*
Based on the final state, which statement is true about the substances in the beakers?
The final substances in both beakers are mixtures.
The final substances in both beakers are pure substances.
The final substance in Beaker A is a pure substance and in Beaker B is a mixture.
The final substance in Beaker A is a mixture and in Beaker B is a pure substance.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
Questions





Mathematics, 18.04.2020 06:42

Mathematics, 18.04.2020 06:42

Social Studies, 18.04.2020 06:42


Geography, 18.04.2020 06:42

English, 18.04.2020 06:42

Mathematics, 18.04.2020 06:42

Mathematics, 18.04.2020 06:42


Mathematics, 18.04.2020 06:42

Social Studies, 18.04.2020 06:42

Mathematics, 18.04.2020 06:42

Mathematics, 18.04.2020 06:42


Mathematics, 18.04.2020 06:43



