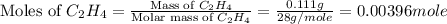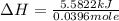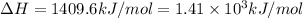Chemistry, 07.04.2020 15:12 kimberlylove387
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of ethylene (C 2H 4) was burned in this calorimeter, the temperature increased by 2.26 K. Calculate the energy of combustion for one mole of ethylene. a. -50.3 kJ/mol b. -1.41 x 103 kJ/mol c. -0.274 kJ/mol d. -624 kJ/mol e. -5.29 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of et...
Questions

Social Studies, 19.05.2021 14:00

Mathematics, 19.05.2021 14:00


Mathematics, 19.05.2021 14:00


Arts, 19.05.2021 14:00


Geography, 19.05.2021 14:00




Mathematics, 19.05.2021 14:00


Mathematics, 19.05.2021 14:00


Social Studies, 19.05.2021 14:00

English, 19.05.2021 14:00

Mathematics, 19.05.2021 14:00

Social Studies, 19.05.2021 14:00

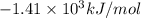
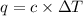
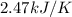
 = Change in temperature = 2.26 K
= Change in temperature = 2.26 K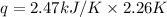
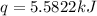
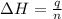
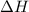 = energy of combustion for one mole of ethylene = ?
= energy of combustion for one mole of ethylene = ?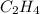 = 0.111 g
= 0.111 g