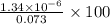Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Solid NaBr is slowly added to a solution that is 0.073 M in Cu+ and 0.073 M in Ag+.Which compound wi...
Questions

Social Studies, 23.09.2019 04:30

Social Studies, 23.09.2019 04:30

Mathematics, 23.09.2019 04:30

Biology, 23.09.2019 04:30

Mathematics, 23.09.2019 04:30




Mathematics, 23.09.2019 04:30

English, 23.09.2019 04:30

English, 23.09.2019 04:30


Chemistry, 23.09.2019 04:30

Mathematics, 23.09.2019 04:30






English, 23.09.2019 04:30

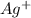 when CuBr just begins to precipitate is,
when CuBr just begins to precipitate is, 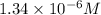
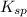 for CuBr is
for CuBr is 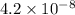
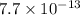
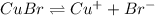
![K_{sp}=[Cu^+][Br^-]](/tpl/images/0586/4611/12867.png)
![4.2\times 10^{-8}=0.073\times [Br^-]](/tpl/images/0586/4611/29ab2.png)
![[Br^-]=5.75\times 10^{-7}M](/tpl/images/0586/4611/05d81.png)
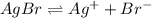
![K_{sp}=[Ag^+][Br^-]](/tpl/images/0586/4611/90aa5.png)
![7.7\times 10^{-13}=[Ag^+]\times 5.75\times 10^{-7}M](/tpl/images/0586/4611/053c9.png)
![[Ag^+]=1.34\times 10^{-6}M](/tpl/images/0586/4611/7e622.png)