
Chemistry, 07.04.2020 17:03 ashley110608
Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the statements about reaction A and reaction B are true? Reaction B is likely to occur at a faster rate than reaction A. Reaction A is more likely to occur at all than reaction B. Reaction B is more likely to occur at all than reaction A. Reaction A is likely to occur at a faster rate than reaction B.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the...
Questions


Biology, 29.08.2019 18:30

Health, 29.08.2019 18:30


Mathematics, 29.08.2019 18:30

English, 29.08.2019 18:30


Mathematics, 29.08.2019 18:30



History, 29.08.2019 18:30

Mathematics, 29.08.2019 18:30


Biology, 29.08.2019 18:30



Physics, 29.08.2019 18:30


Chemistry, 29.08.2019 18:30

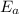 , and it is measured in Joules (J), KiloJoules (KJ) or Kilocalories per mole (Kcal/mol)
, and it is measured in Joules (J), KiloJoules (KJ) or Kilocalories per mole (Kcal/mol)

