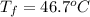Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Aforce of attraction/repulsion due to the spin of electrons what is this force?
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
F 100. grams of liquid water at 100.°C and 200. grams of water at 20.0°C are mixed in an insulated c...
Questions


Social Studies, 13.12.2021 17:00

Mathematics, 13.12.2021 17:00

SAT, 13.12.2021 17:00

Mathematics, 13.12.2021 17:00



SAT, 13.12.2021 17:00


SAT, 13.12.2021 17:00

SAT, 13.12.2021 17:00

Mathematics, 13.12.2021 17:00




SAT, 13.12.2021 17:00


Social Studies, 13.12.2021 17:00

Mathematics, 13.12.2021 17:00

Biology, 13.12.2021 17:00

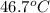
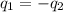
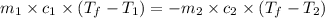
 =
= 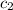 = specific heat of liquid water = Same
= specific heat of liquid water = Same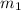 = mass of liquid water = 100 g
= mass of liquid water = 100 g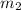 = mass of water = 200 g
= mass of water = 200 g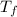 = final temperature of mixture = ?
= final temperature of mixture = ?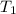 = initial temperature of liquid water =
= initial temperature of liquid water = 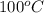
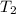 = initial temperature of water =
= initial temperature of water = 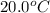
![(100g)\times (T_f-100.0)^oC=-[(200g)\times (T_f-20.0)^oC]](/tpl/images/0586/5833/2dfce.png)