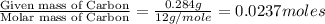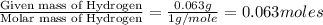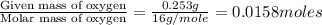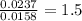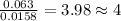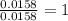Chemistry, 07.04.2020 17:26 Crtive6538
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produced 1.043 g of CO2 and 0.5670 g of H2O. What is the empirical formula of the compound? Note because you are not able to enter subscripts enter the answer in the form: CxHyOz

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produc...
Questions


Social Studies, 06.12.2021 01:00

Mathematics, 06.12.2021 01:00

Mathematics, 06.12.2021 01:00





Mathematics, 06.12.2021 01:00


Mathematics, 06.12.2021 01:00

Mathematics, 06.12.2021 01:00

Spanish, 06.12.2021 01:00


Mathematics, 06.12.2021 01:00

English, 06.12.2021 01:00

Biology, 06.12.2021 01:00


Mathematics, 06.12.2021 01:00

Mathematics, 06.12.2021 01:00

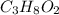
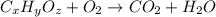
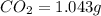
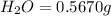
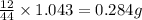 of carbon will be contained.
of carbon will be contained.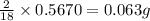 of hydrogen will be contained.
of hydrogen will be contained.