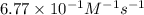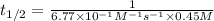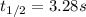Chemistry, 07.04.2020 18:45 ellycleland16
"The elementary reaction 2 NO2(g) → 2 NO(g) + O2(g) is second order in NO2 and the rate constant at 600 K is 6.77 × 10-1 M-1s-1. The reaction half-life at this temperature when [NO2]0 = 0.45 M is s."

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
"The elementary reaction 2 NO2(g) → 2 NO(g) + O2(g) is second order in NO2 and the rate constant at...
Questions




Mathematics, 02.02.2021 21:20



Mathematics, 02.02.2021 21:20



Mathematics, 02.02.2021 21:20

Mathematics, 02.02.2021 21:20

Biology, 02.02.2021 21:20

English, 02.02.2021 21:20

Arts, 02.02.2021 21:20





Mathematics, 02.02.2021 21:20

Mathematics, 02.02.2021 21:20

![t_{1/2}=\frac{1}{k\times [A_o]}](/tpl/images/0586/7504/a9a58.png)
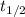 = half-life = ?
= half-life = ?![[A_o]](/tpl/images/0586/7504/dc622.png) = initial concentration = 0.45 M
= initial concentration = 0.45 M