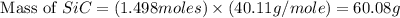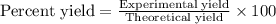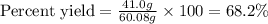Equation: SiO2 + 3C = SiC + 2CO When 90.0 g of silicon dioxide is heated with an excess of carbon, 41.0 g of silicon carbide is produced. What is the percent yield of this reaction? (find the theoretical amount of SiC using stoichiometry, then calculate percent yield)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

You know the right answer?
Equation: SiO2 + 3C = SiC + 2CO When 90.0 g of silicon dioxide is heated with an excess of carbon, 4...
Questions





Spanish, 06.09.2019 16:20


History, 06.09.2019 16:20




Mathematics, 06.09.2019 16:20

Biology, 06.09.2019 16:20

Mathematics, 06.09.2019 16:20







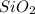 = 90.0 g
= 90.0 g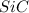 = 41.0 g
= 41.0 g

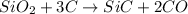
 react to give 1.498 mole of
react to give 1.498 mole of