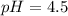Chemistry, 07.04.2020 20:01 lmcginnis2003
Calculate the pH of a solution prepared by dissolving 0.15 mol benzoic acid (C7H5O2H) and 0.30 mol of sodium benzoate (Na C7H5O2) in water sufficient to yield 1.00 L of solution. The Ka of benzoic acid is 6.50 x 10-5

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Calculate the pH of a solution prepared by dissolving 0.15 mol benzoic acid (C7H5O2H) and 0.30 mol o...
Questions

Mathematics, 13.01.2021 22:00


Mathematics, 13.01.2021 22:00

Biology, 13.01.2021 22:00

Mathematics, 13.01.2021 22:00

History, 13.01.2021 22:00

Mathematics, 13.01.2021 22:00




Mathematics, 13.01.2021 22:00


Mathematics, 13.01.2021 22:00






Mathematics, 13.01.2021 22:00

Mathematics, 13.01.2021 22:00

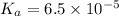
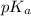 .
.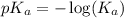
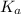 in this expression, we get:
in this expression, we get: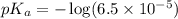
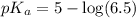
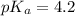
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0587/0629/e961a.png)
![pH=4.2+\log [\frac{(\frac{0.30}{1.00L})}{(\frac{0.15}{1.00L})}]](/tpl/images/0587/0629/17ffa.png)