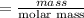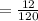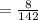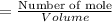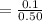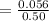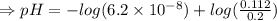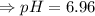Chemistry, 07.04.2020 20:14 raekwonpowell10
6. What is the pH of the buffer that results when 12.0 g of NaH2PO4 and 8.00 g of Na2HPO4 are diluted with water to a volume of 0.50 L? (Ka of H2PO4– = 6.2 x10–8, the molar masses of NaH2PO4 and Na2HPO4 are 120.0 g/mol and 142.0 mol, respectively)

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2
You know the right answer?
6. What is the pH of the buffer that results when 12.0 g of NaH2PO4 and 8.00 g of Na2HPO4 are dilute...
Questions


Mathematics, 29.04.2021 18:20

Health, 29.04.2021 18:20


Mathematics, 29.04.2021 18:20


Biology, 29.04.2021 18:20


English, 29.04.2021 18:20

Biology, 29.04.2021 18:20

Mathematics, 29.04.2021 18:20


Physics, 29.04.2021 18:20

Social Studies, 29.04.2021 18:20

English, 29.04.2021 18:20

Spanish, 29.04.2021 18:30

Mathematics, 29.04.2021 18:30

Business, 29.04.2021 18:30

Biology, 29.04.2021 18:30

Chemistry, 29.04.2021 18:30

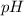 of the buffer solution is 6.96.
of the buffer solution is 6.96.![pH= pK_a+log \frac{[salt]}{[acid]}](/tpl/images/0587/1250/39469.png) .
.